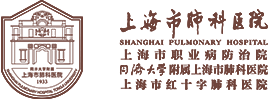The Department of Thoracic Surgery of Shanghai Pulmonary Hospital is actively engaged in clinical research
Scope of drug clinical studies in Thoracic Surgery Department
The clinical study team of Thoracic Surgery Department of Shanghai Pulmonary Hospital, relying on the strong medical and scientific research strength of Shanghai Pulmonary Hospital and Thoracic Surgery Department of Shanghai Pulmonary Hospital, has conducted 107 clinical trials of various drugs since 2018. As of May 2023, the Department of Thoracic Surgery of Shanghai Pulmonary Hospital, as the main center or sub-center, has conducted a total of 40 GCP drug clinical trials, with studies covering:
1. neoadjuvant and adjuvant therapy for locally advanced non-small cell lung cancer that is negative for driver mutations;
2. neoadjuvant and adjuvant therapy for locally advanced non-small cell lung cancer that is positive for driver mutations;
3. first-, second-, and late-line therapy for metastatic or recurrent non-small cell lung cancer;
4. first-, second-, and subsequent-line therapy for extensive-stage small cell lung cancer.
The Thoracic Surgery Clinical Research Team at Shanghai Pulmonary Hospital is also concurrently conducting a series of 67 investigator-initiated prospective clinical studies (IITs) covering:
1. postoperative adjuvant therapy for driver-negative non-small cell lung cancer;
2. postoperative adjuvant therapy for kinesin-positive non-small cell lung cancer;
3. neoadjuvant immunotherapy for kinesin-negative locally advanced non-small cell lung cancer;
4. neoadjuvant targeted therapy for kinesin-positive locally advanced non-small cell lung cancer;
5. neoadjuvant immunotherapy for locally advanced small cell lung cancer;
6. clinical studies that include the treatment of rare thoracic malignant diseases such as tracheal tumors, thymic tumors, and malignant pleural mesothelioma.
Subject Recruitment for Thoracic Drug Clinical Studies
With the stunning results published in the ADAURA study, ositinib has become the first choice for postoperative adjuvant therapy for patients with EGFR mutation-positive NSCLC. The clinical research team of the Department of Thoracic Surgery of Shanghai Pulmonary Hospital, led by Prof. Chen Chang, Prof. Zhang Peng, and Prof. Hu Xuefei, has carried out a number of prospective clinical studies on neoadjuvant EGFR-TKI-targeted therapy. Among them, LungMate-012 is a prospective, phase II, randomized controlled clinical study to enroll patients with locally advanced EGFR mutation-positive NSCLC with the possibility of radical resection, and to evaluate the safety and efficacy of neoadjuvant third-generation EGFR-TKI in combination with bevacizumab for locally advanced EGFR mutation-positive NSCLC.
With the publication of data from clinical studies such as CheckMate-816, KeyNote-091, and KeyNote-671, anti-PD-1 inhibitors have been widely used in neoadjuvant and adjuvant treatment of NSCLC. Under the guidance and direction of Prof. Chen Chang, Prof. Gerning Jiang, Prof. Zhang Peng, and Prof. Yuming Zhu, the clinical research team of the Department of Thoracic Surgery of Shanghai Pulmonary Hospital has carried out a number of clinical studies on neoadjuvant and/or adjuvant immunotherapy. Among them, LungMate-013 study has been initiated at the beginning of this year, and now subject enrollment is under recruitment, which intends to enroll unresectable stage III NSCLC patients, and intends to evaluate the conversion rate of induction regimen of anti-PD-1 inhibitor combined with chemotherapy for unresectable stage III NSCLC patients with curative downstaging and operable disease; and also to compare the efficacy of radical pneumonectomy versus radical radiotherapy for curative operable patients. Efficacy.
It is a great success for anti-PD-L1 inhibitors in the treatment of limited and extensive stage small cell lung cancer. The clinical research team of the Department of Thoracic Surgery of Shanghai Pulmonary Hospital has conducted several prospective clinical studies on neoadjuvant immunotherapy in combination with radical lung cancer surgery for the treatment of limited-stage small cell lung cancer. Among them is the LungMate-015 study, which is intended to enroll patients with limited-stage small-cell lung cancer who have the potential for radical resection, and to evaluate the safety and efficacy of a multidisciplinary integrated treatment model of neoadjuvant induction therapy with anti-PD-L1 inhibitors in combination with platinum-containing two-agent chemotherapy, followed by either radical pneumonectomy or radical radiotherapy.
The above clinical study is in the stage of subject enrollment, and we look forward to recruiting more eligible subjects, providing research-proven safe and efficient neoadjuvant treatment options, providing greater survival benefits, and giving higher quality medical services to the subjects, and bringing more benefits to the subjects.
Representative results of thoracic surgery drug clinical research
LungMate-001 is the first prospective, phase II, single-arm clinical study related to neoadjuvant immunotherapy conducted by the co-principal investigator of the Department of Thoracic Surgery of our Center, Prof. Jiang Gening and Prof. Zhang Peng, with the assistance of Prof. Chen Chang and Prof. Zhu Yuming. The study has been officially launched since September 2019, and it lasted for two years, with the enrollment of 50 patients in total with resectable locally advanced driver mutation-negative non-small cell lung cancer subjects undergoing neoadjuvant immune-combination chemotherapy followed by combined radical lung cancer surgery. The study aims to explore the safety and efficacy of novel treatment modalities in the field of neoadjuvant therapy for non-small cell lung cancer. The results of the study suggest that the neoadjuvant regimen of sindilizumab combined with platinum-containing two-agent chemotherapy can significantly improve the primary pathological remission rate (MPR: 43.3%) and the 1-year recurrence-free survival rate of all subjects is nearly 90% compared with the traditional neoadjuvant regimen of platinum-containing two-agent chemotherapy. The neoadjuvant sindilizumab combined with platinum-containing two-agent chemotherapy was finally confirmed to be safe and feasible for the treatment of locally advanced operable non-small cell lung cancer. The research results have been published in The Annals of Thoracic Surgery, a core journal of thoracic surgery, marking the thoracic surgery department of Shanghai Pulmonary Hospital has stepped on a new level in the field of IIT.
LungMate-002 is another prospective, phase II, single-arm clinical study related to neoadjuvant immunotherapy conducted by the clinical research team of the Department of Thoracic Surgery of Shanghai Pulmonary Hospital, based on the successful advancement of LungMate-001. It aims to evaluate the feasibility of radical lung cancer surgery for potentially resectable locally advanced non-small cell lung cancer after receiving down-staging neoadjuvant therapy with anti-PD-1 monoclonal antibody combined with platinum-containing two-agent chemotherapy; and to explore the predictors of efficacy and transcriptomic features associated with immunotherapy. The study was conducted by Prof. Jiang Gening, Prof. Zhang Peng and Prof. Zhu Yuming of the Department of Thoracic Surgery, and included 12 patients with locally advanced resectable non-small cell lung cancer and 38 patients with locally advanced potentially resectable non-small cell lung cancer. The results of the study suggested that the objective remission rate of subjects treated with neoadjuvant therapy via teraplizumab combined with platinum-containing two-agent chemotherapy was 76%, and the primary pathological remission rate of surgical patients was as high as 55.6%, and the 1-year survival rate of all subjects was over 90%. It is worth mentioning that this study confirmed that after neoadjuvant treatment, nearly 70% of patients with unresectable locally advanced NSCLC were given the opportunity to have their lung cancer eradicated, and its high surgical conversion and R0 resection rates initially confirmed the feasibility of neoadjuvant immunization in combination with chemotherapy for the treatment of potentially resectable NSCLC. And transcriptome sequencing of tumor tissues before and after treatment of subjects included in the study revealed that patients with high expression of chitosanase 3-like protein 1 (CHI3L1) showed a trend towards longer survival and is a reliable biomarker related to the efficacy of immunotherapy. The research results have been published in the leading medical journal BMC Medicine in 2022.
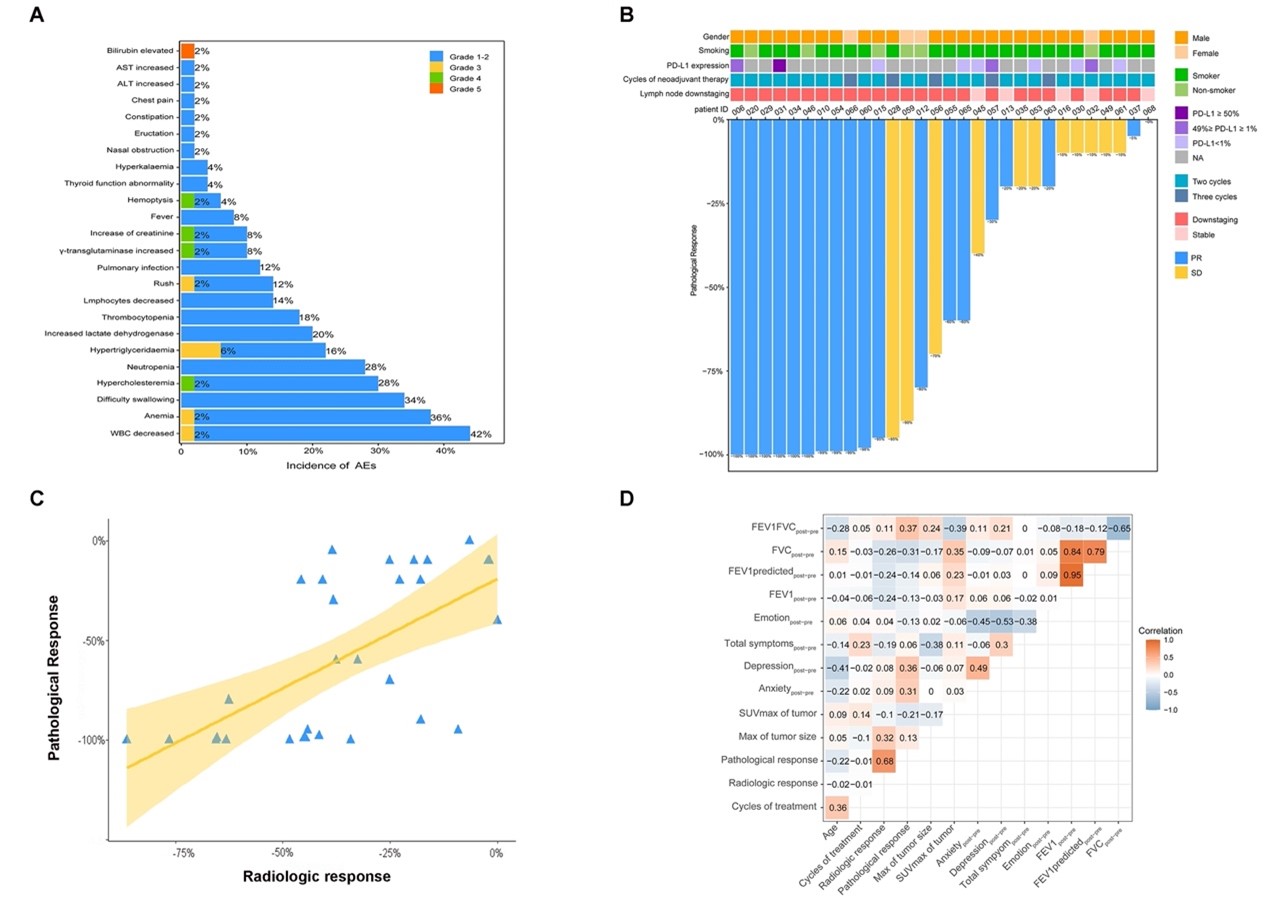
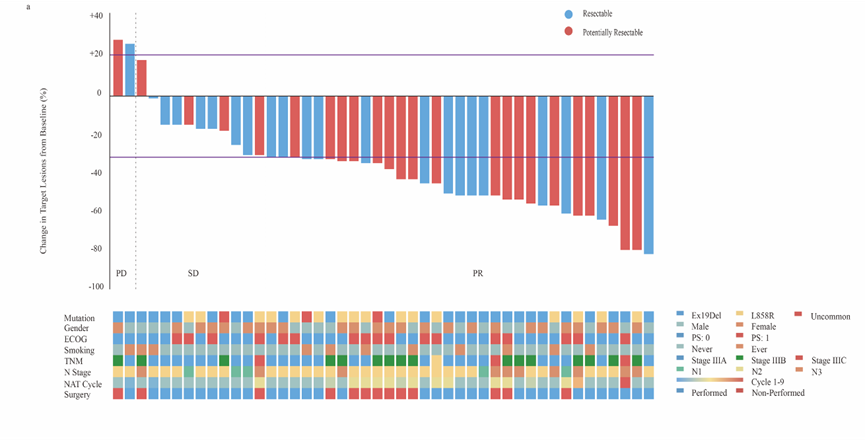
Lungate-004 is a prospective, phase II, single-arm clinical study of neoadjuvant EGFR-TKI-targeted therapy for locally advanced EGFR mutation-positive patients conducted by the clinical research team of the Department of Thoracic Surgery of Shanghai Pulmonary Hospital. It aims to assess the feasibility of radical lung cancer surgery for locally advanced EGFR mutation-positive non-small cell lung cancer after neoadjuvant monotherapy with afatinib, a second-generation EGFR-TKI; and to explore the efficacy predictors and transcriptomic features associated with targeted therapy. The study was conducted by Prof. Jiang Gening and Prof. Zhang Peng of the Department of Thoracic Surgery. This study confirmed that: the objective remission rate after neoadjuvant EGFR-TKI targeted therapy is over 70%; 56% of stage III unresectable EGFR mutation-positive NSCLC patients were offered the opportunity of radical surgery after afatinib induction therapy; the 1-year survival rate of treated patients is 100%, and the 1-year progression-free survival rate is more than 95%; and the rate of treatment-related adverse events is low, with a favorable safety profile. The research results have been orally reported at the 59th The Society of Thoracic Surgeons Conference. Meanwhile, this study has entered the research report writing stage.
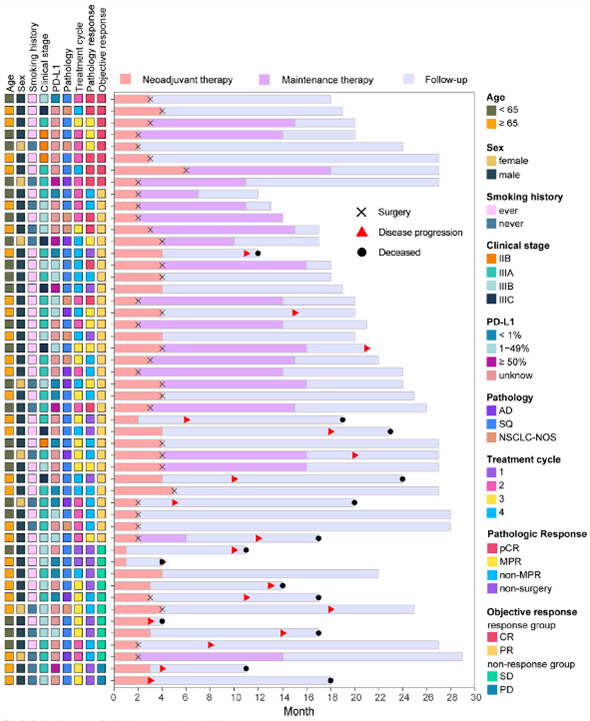
The Department has substantially improved the treatment effect of the subjects in the process of conducting clinical trials, greatly benefited the subjects in their rights and interests, and comprehensively improved the quality of medical services. Meanwhile, the clinical research team of the Department of Thoracic Surgery of Shanghai Pulmonary Hospital actively explores new and refined treatment modes for various benign and malignant diseases in the chest, explores new biomarkers related to the efficacy of various treatments, and analyzes the changes in the microenvironment induced by various treatment modes at the level of molecular biology, and the relevant research results have been published one after another in authoritative journals of domestic and foreign medical science.
Latest articles
More- Nat Commun | Phase II clinical trial by Profs. Jiang Gening and Zhang Peng reveals safety and efficacy of neoadjuvant afatinib in EGFR-mutant non-small cell lung cancer and identifies new therapeutically sensitive biomarkers 2023-12-06
- Professor Zhang Peng's team has elucidated the molecular mechanism by which intratumoral fungal microorganisms affect the progression of lung adenocarcinoma 2023-12-06
- Int J Surg | Prof. Chen Chang's team elucidates the prognostic significance of lymph node staging based on the number of metastases 2023-12-06
- STTT | Chen Chang, Tongji University / Fang Wenjie/ Pan Weihua, Naval Medical University: Key mechanisms of gut microbes and lung transplantation stability revealed 2023-11-30
- The Department of Thoracic Surgery of Shanghai Pulmonary Hospital is actively engaged in clinical research 2023-11-30
- Introduction to Artificial Intelligence Multi-Organomics Series Research Achievements 2023-11-30
- BMC Med | Professor Zhang Peng: Treprostinil neoadjuvant therapy for stage II-III NSCLC gains further international recognition 2023-11-30
- Introduction to Surgical Laboratory 2023-11-30