BMC Med | Professor Zhang Peng: Treprostinil neoadjuvant therapy for stage II-III NSCLC gains further international recognition
Introduction:
On December 30, 2022, the full text of the LungMate 002 study[1] conducted by the team of Prof. Jiang Gening, Prof. Zhu Yuming, and Prof. Zhang Peng from Shanghai Pulmonary Hospital affiliated with Tongji University was published in the journal of BMC Medicine (IF = 11.15), which showed that neoadjuvant treatment of Stage II-III NSCLC with teraplizumab combined with chemotherapy Lung Cancer (NSCLC) patients had an objective remission rate (ORR) of 76.0%, and all patients who underwent surgery achieved R0 resection (100%), with a major pathologic remission rate (MPR) as high as 55.6%, and with safe and controllable adverse events. On this important occasion, we invited Prof. Jiang Gening, Prof. Zhu Yuming and Prof. Zhang Peng to introduce and interpret this study to guide clinical practice.
Research Background
Lung cancer is one of the most prevalent malignant tumors worldwide and the leading cause of cancer-related death. However, conventional radiotherapy regimens provide limited benefit to patients, and targeted therapies are only effective for some sensitive patients. In recent years, several clinical trials of programmed cell death receptor 1 (PD-1) and programmed cell death ligand 1 (PD-L1) checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) have been conducted, confirming their efficacy in neoadjuvant as well as adjuvant treatment regimens.
Teraplizumab is the first originator PD-1 inhibitor to be marketed in China and has been approved for first-line treatment of advanced driver gene-negative NSCLC in combination with standard chemotherapy. This was used in the LungMate 002 study to explore the safety and efficacy of neoadjuvant treatment with teraplizumab in combination with chemotherapy in the treatment of stage II-III NSCLC, to demonstrate its feasibility in enabling surgery after downstaging of some potentially resectable NSCLC, and to characterize the predictors of efficacy and transcriptomic features during treatment.
Study Methods
LungMate 002 is an investigator-initiated, open-label, single-arm, phase II clinical trial. It primarily enrolls patients with first-time treatment, EGFR/ALK wild-type, stage II-III NSCLC. Patients receive 2-4 cycles of teraplizumab (240 mg, q3w) in combination with carboplatin-based chemotherapy every 21 days, reevaluated for surgery after 2 cycles of treatment, and treated surgically if complete resection is acceptable, otherwise continued for the remainder of the treatment cycle, with adjuvant chemotherapy 4-6 weeks postoperatively, and adjuvant immunotherapy at the patient's discretion and continuing for 1 year, and with a review of baseline period and RNA sequencing analysis of tumor samples and lymph node samples during treatment. The primary study endpoints were safety and major pathologic remission (MPR) rate, secondary endpoints were R0 resection rate, 5-year progression-free survival (PFS) rate, and 5-year overall survival (OS) rate, and exploratory endpoints were treatment-related predictive biomarkers and transcriptome characterization.
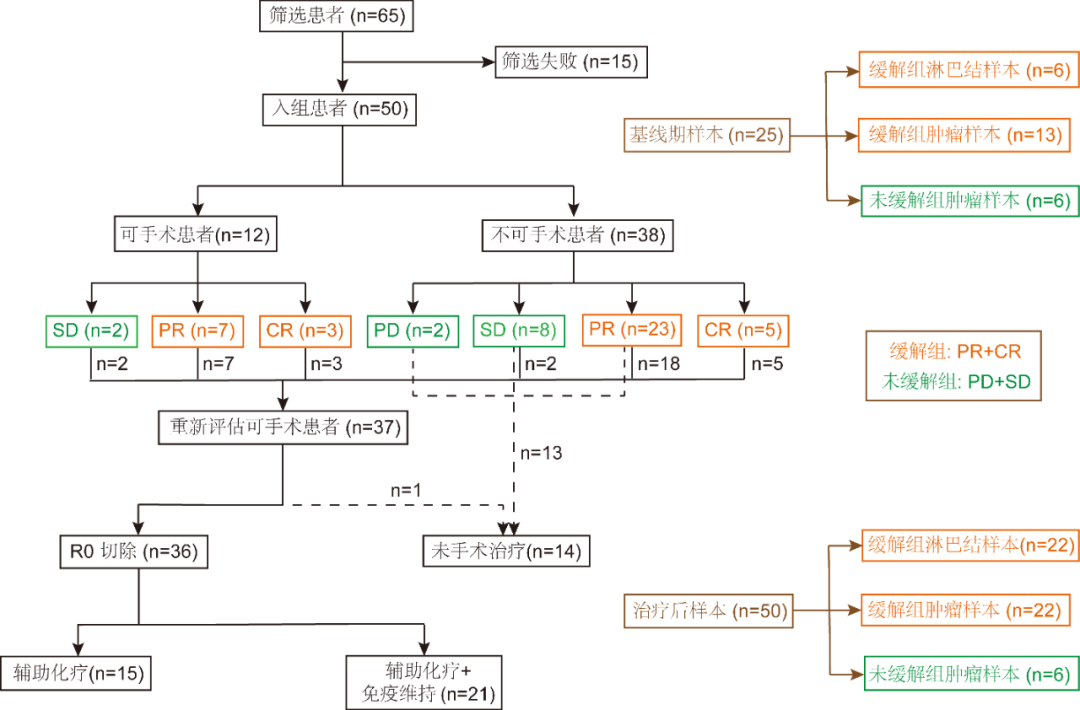
Figure 1. Flowchart of patient enrollment and sequencing sample collection
Study Results
Patient baseline characteristics:
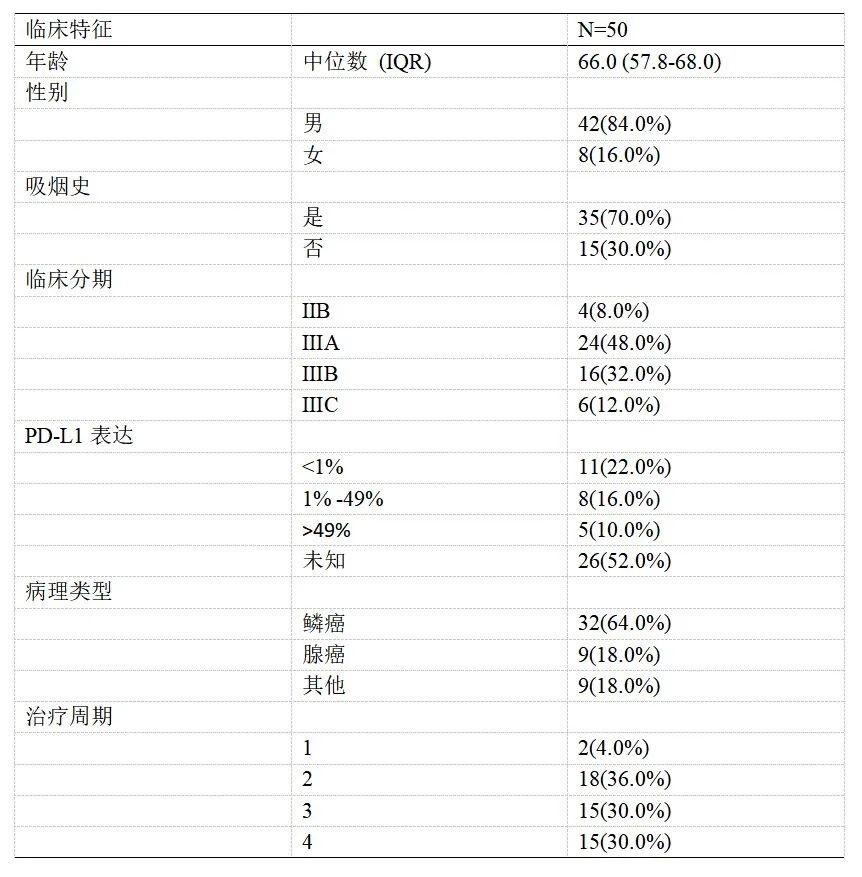
A total of 50 eligible patients were enrolled in the clinical trial, including 12 (24%) patients who were operable prior to neoadjuvant therapy versus 38 (76%) patients who were not operable for treatment; clinical characteristics are shown in Table 1.
Table 1 Clinical characteristics of patients
Safety outcomes:
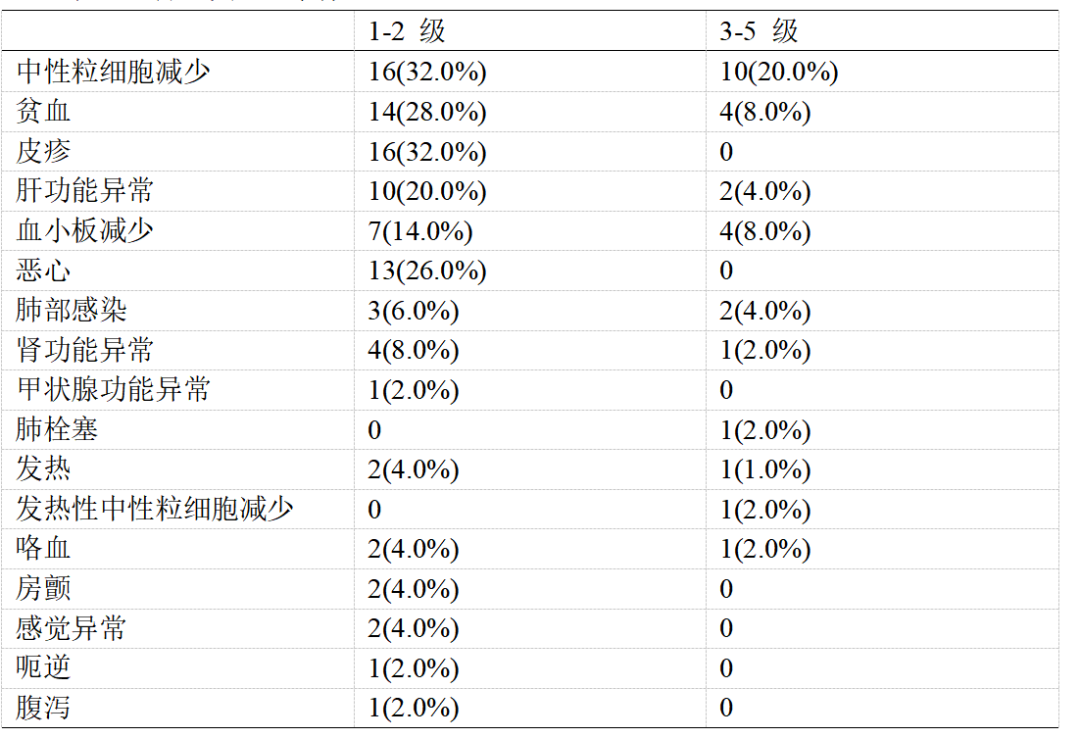
During treatment, 48 (96.0%) patients experienced treatment-related adverse events (TRAEs). Of these, grade 1-2 TRAEs occurred in 31 (62.0%) patients and grade 3-5 TRAEs occurred in 17 (34.0%) patients. The most common grade 1 or 2 TRAEs were neutropenia (16, 32.0%), rash (16, 32%) and anemia (14, 28.0%). (See Table 2 for details).
Table 2 Treatment-related adverse events
Neoadjuvant efficacy outcomes:
After the last 1 neoadjuvant cycle, 37 (74.0%) patients were evaluated as eligible for surgery, including all 12 (100.0%) operable patients and 25 (68.4%) inoperable patients, with 1 (2%) of the pre-treatment inoperable patients declining surgery due to concerns about potential risks. Thus, 36 (72.0%) patients ultimately underwent surgery and R0 resection was performed in all patients.
The objective remission rate of patients was 76.0% (38/50), of which 8 (16.0%) patients were in complete remission (CR) and 30 (60.0%) patients were in partial remission (PR). Of the 36 patients who underwent surgery, 23 (63.9%) achieved lymph node downstaging, and 20 (55.6%) patients realized MPR, including 10 (27.8%) patients who were pCR. (See Table 3 for details).
Latest articles
More- Nat Commun | Phase II clinical trial by Profs. Jiang Gening and Zhang Peng reveals safety and efficacy of neoadjuvant afatinib in EGFR-mutant non-small cell lung cancer and identifies new therapeutically sensitive biomarkers 2023-12-06
- Professor Zhang Peng's team has elucidated the molecular mechanism by which intratumoral fungal microorganisms affect the progression of lung adenocarcinoma 2023-12-06
- Int J Surg | Prof. Chen Chang's team elucidates the prognostic significance of lymph node staging based on the number of metastases 2023-12-06
- STTT | Chen Chang, Tongji University / Fang Wenjie/ Pan Weihua, Naval Medical University: Key mechanisms of gut microbes and lung transplantation stability revealed 2023-11-30
- The Department of Thoracic Surgery of Shanghai Pulmonary Hospital is actively engaged in clinical research 2023-11-30
- Introduction to Artificial Intelligence Multi-Organomics Series Research Achievements 2023-11-30
- BMC Med | Professor Zhang Peng: Treprostinil neoadjuvant therapy for stage II-III NSCLC gains further international recognition 2023-11-30
- Introduction to Surgical Laboratory 2023-11-30